The negotiation of medical insurance access is an important part of the adjustment of the national medical insurance catalogue. It can not only ensure the high-quality development of the basic medical security system, but also enable the majority of insured patients to enjoy new drugs on the domestic market at a lower price, including new drugs with curative effects for major diseases such as cancer and rare diseases.
Trend: Centralized purchase and normalization of medical insurance negotiation, and the decrease of new exclusive varieties tends to be stable
In terms of access mode, China has gradually turned to the orientation of product and price advantages. Drugs with advantages in products, clinics and prices are more competitive in access.
With the continuous promotion of the consistency evaluation of generic drugs, the National Medical Insurance Bureau has carried out seven batches of eight rounds of centralized procurement of drugs in quantity. The first five batches of centralized procurement involved 234 varieties, with an average decrease of 52-59%. The sixth batch of insulin was selected from 42 products, with an average decrease of 48%. The seventh batch of centralized purchase of 60 products was selected, with an average decrease of 48%.
At present, China has conducted a total of six rounds of medical insurance drug negotiations for innovative drugs. The average decline of innovative drugs selected for the first time is basically 40-62%. After the majority of innovative drugs entered the medical insurance, they realized the exchange of price for volume, and the sales volume increased significantly.

《谈判药品续约规则》规则二的“简易续约”降幅规则

表 2022年医保目录调整三种谈判药品续约规则
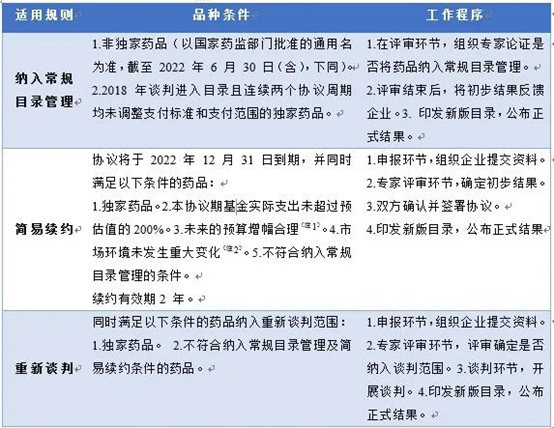
Note 1: The future budget increase reasonably includes two situations: (1) drugs without adjusting the payment scope: the increase of the fund expenditure budget in the next two years shall not exceed 100% (compared with the higher of the fund expenditure budget and the actual fund expenditure in this agreement period, the same below). (2) Drugs with adjusted payment scope: if the original payment scope meets the conditions (1), the increase of fund expenditure budget in the next two years due to the adjustment of payment scope shall not exceed 100%.
Note 2: "Significant change" mainly refers to the obvious high price or treatment cost in the same treatment field, the actual sales price of the drug at home and abroad or the converted price of the drug as a gift is significantly lower than the current payment standard, and similar competitors have passed the review in this round of adjustment and may have a significant impact on the price.
Analysis on sales of drugs in China
Under the influence of centralized purchase and national negotiation policies, the sales of national negotiation products in 2021Q2 will still increase significantly in 2019, with a year-on-year increase of 124%. Since the 2018 national negotiation approval has passed the peak of large-scale development, the growth rate is small. The 2020 national negotiation approval has just been implemented in 2021.3, and the large-scale effect is not obvious.

Announcement of the preliminary review results of the 2022 national negotiation
344 of the 490 drugs declared this time passed the preliminary examination. Compared with 2021 (271 of 474 drugs passed the preliminary examination), the number of drugs declared and passed the preliminary form examination has increased to a certain extent. This time, 344 western medicines and Chinese patent medicines passed the preliminary form examination, with 70% passing, including 199 out of the catalog, including 184 western medicines (144 exclusive varieties), 15 Chinese patent medicines (14 exclusive varieties), and 153 newly marketed medicines; There are 145 in the catalogue, including 110 western medicines (88 exclusive varieties), 35 Chinese patent medicines (all exclusive varieties) and 87 newly marketed medicines.

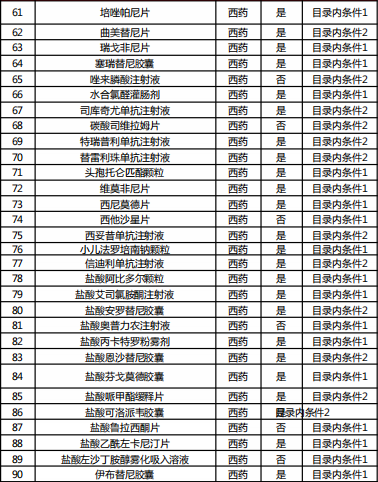
Table 2022 Western medicine and Chinese patent medicine out of the preliminary list of the national negotiation
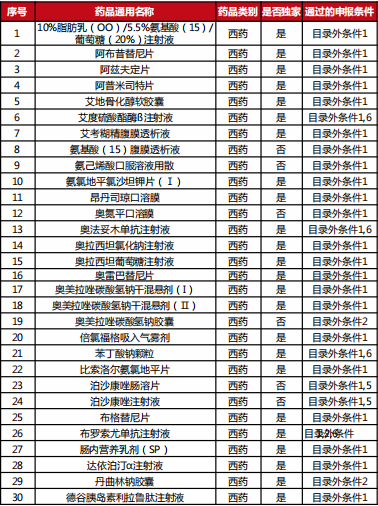
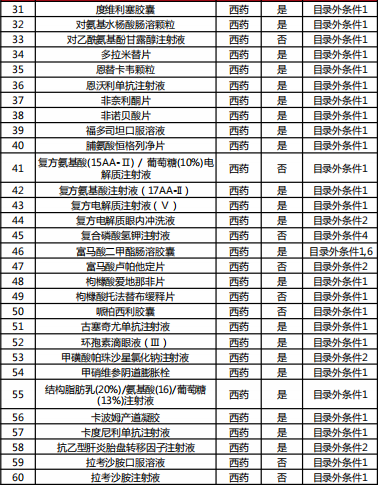
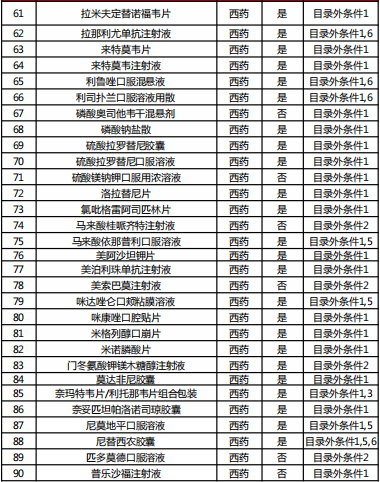
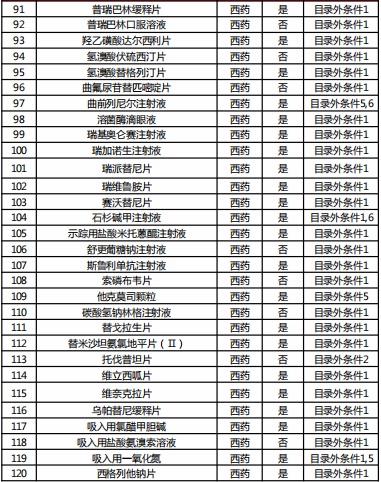
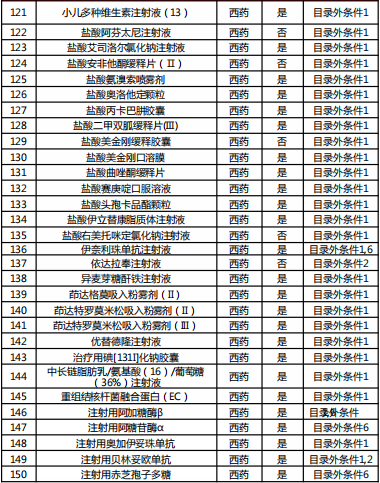

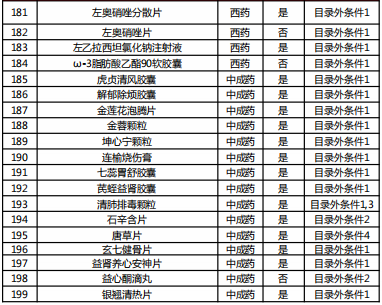
Table Western medicine and Chinese patent medicine in the preliminary review list of the 2022 talks


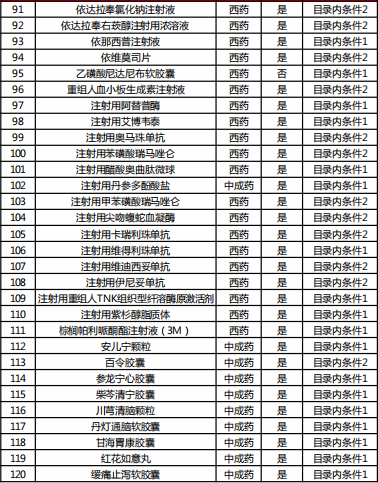
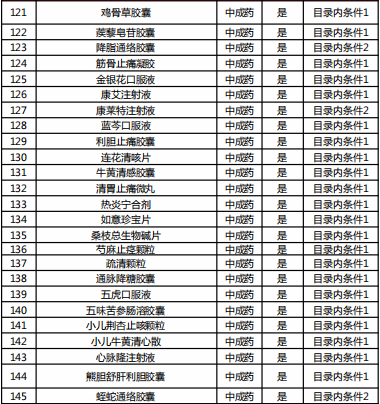
The article comes from Southwest Securities Research and Development Center and the Internet. If there is infringement, please contact to delete
Lindmik Pharmaceutical(Suzhou)Co.,Ltd is a high-tech pharmaceutical enterprise focusing on the research and development, production and sales of innovative pharmaceutical preparations.Equipped with a number of its own innovative R&D platform of dosage forms, including the transdermal drug delivery system, and at the same time, actively introducing the world’s leading nano-based drug delivery, microspheres drug delivery and other cutting-edge pharmaceutical technologies by means of “license in”, the company is a new rapidly developing company pharmaceutical companies that catches people’s eyes.

12th Floor, Building 5, Tianyun Plaza, 111 Wusongjiang Avenue, Guoxiang Street, Wuzhong District, Suzhou City

0512-66020899

0512-66022699

215124