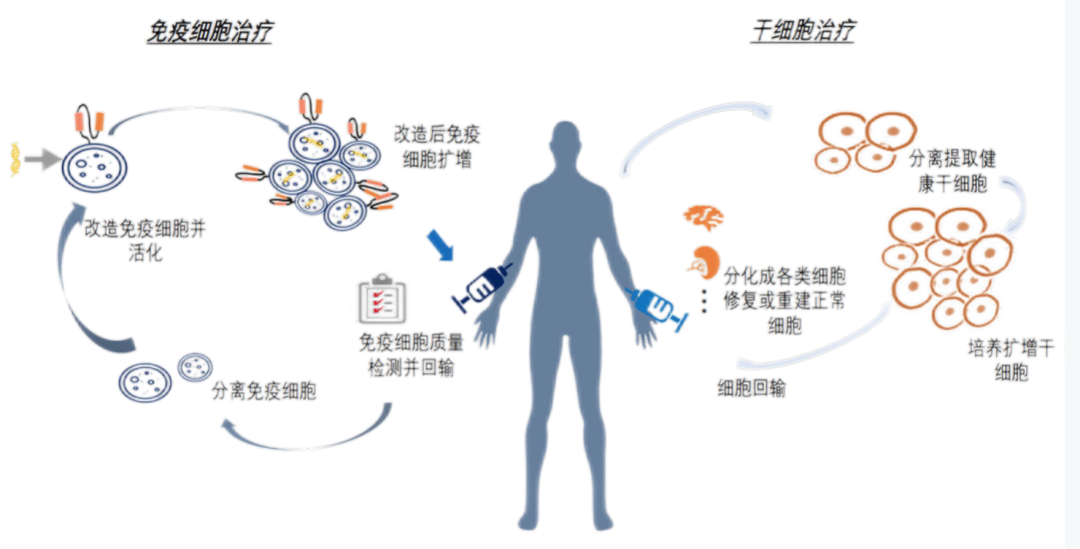
CGT (Cellular and Gene Therapy) refers to the process of transferring the determined genetic material to specific target cells of patients, modifying the expression of individual genes or repairing abnormal genes through gene addition, gene modification, gene silencing, etc., to achieve the goal of curing diseases.
Advantages of cell gene therapy
01 | Highly targeted
The treatment of tumor is highly targeted and has significant effect. It basically does not damage normal tissues when killing cancer cells.
02 | Unique mechanism
It is non cytotoxic, effective, non-toxic and well tolerated.
03 丨 Few side effects and adverse reactions
Compared with traditional surgery, radiotherapy and chemotherapy, there are basically no side effects and adverse reactions, and patients will not suffer during treatment.
Development History
Global drug pipeline under research
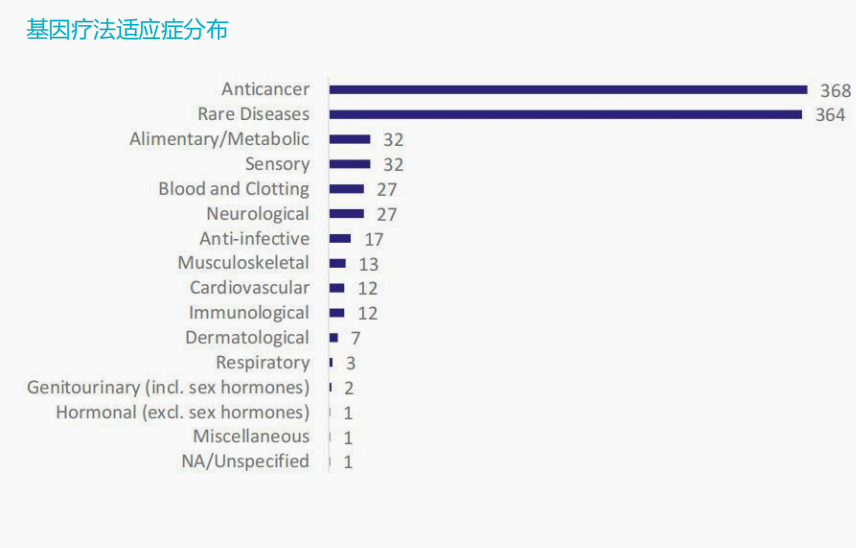
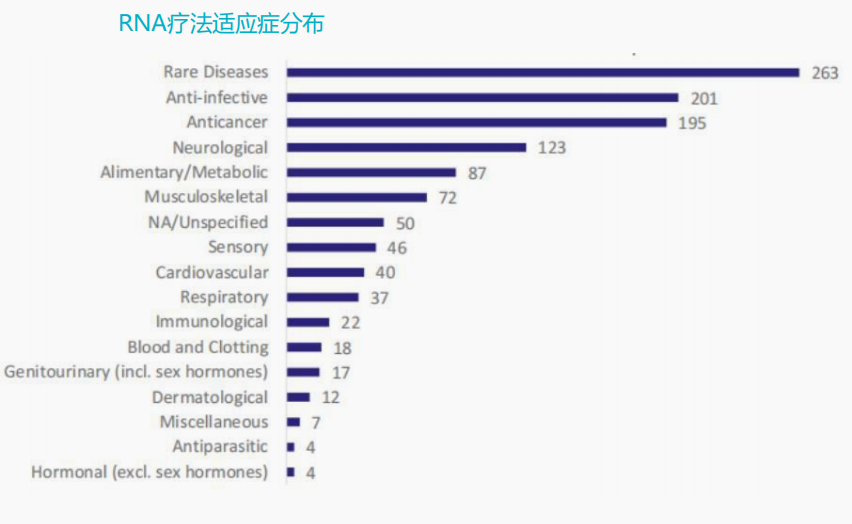
According to ASCGT data, of the 3633 CGT clinical trials in the world, 2024 are gene therapy pipelines, including gene editing of CAR-T therapy, accounting for 55%; 803 items are cell therapy pipelines that do not involve gene editing, accounting for 22%.
Pipeline of drugs under research in China
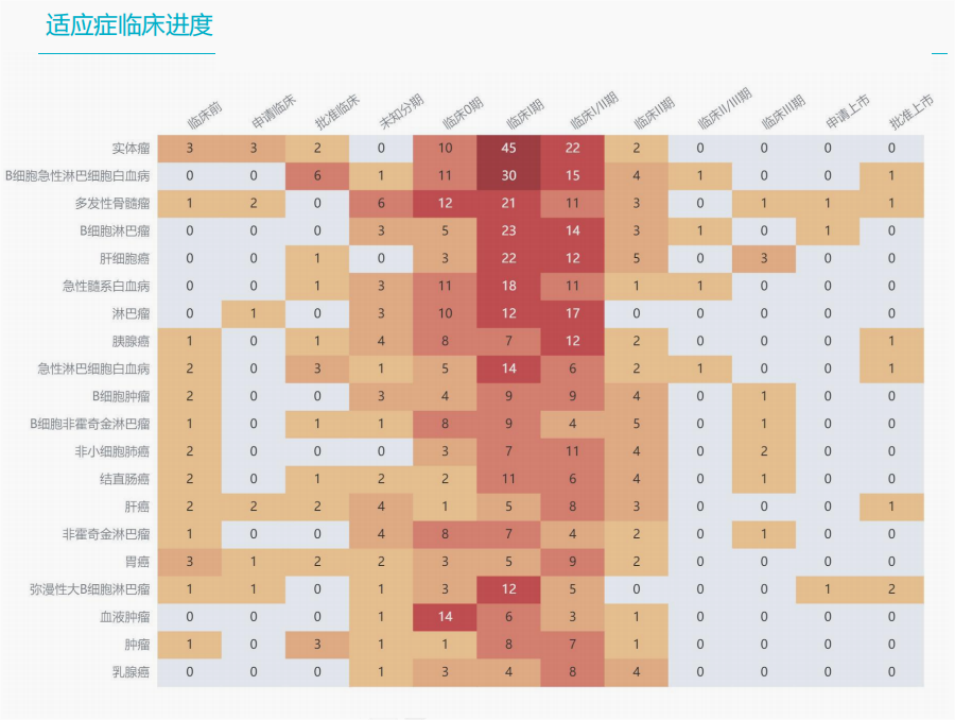
There are more than 700 clinical research pipelines of CGT drugs in China. In addition to 5 listed drugs, there are 2 applications for listing, 259 Phase I clinical projects, 233 Phase II clinical projects, 20 Phase III clinical projects, 26 approved clinical projects, and 52 clinical applications.

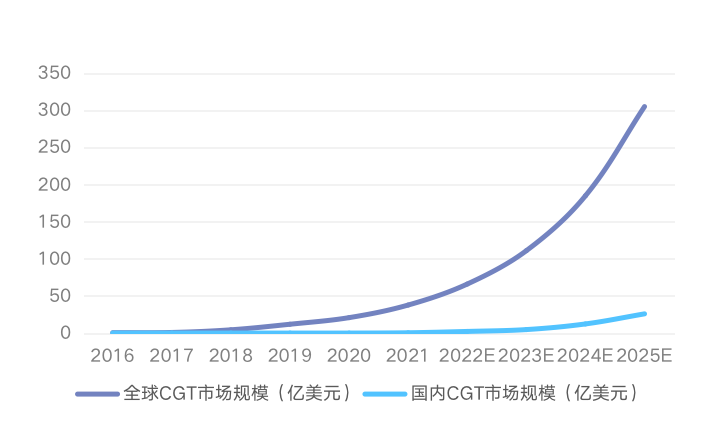
Forecast of global and domestic CGT market size
Data source: Frost Sullivan Consulting (China)'s domestic market in 2016-2020 has a compound annual growth rate of 12.2%, and the market size in 2020 will be 23.8 million yuan. Although the Chinese market started late, it is expected that under the influence of multiple factors such as policy promotion, medical technology progress, and the increase in the number of patients, the track is expected to surge to 17.885 billion yuan in 2025, which has great potential.

CGT regulatory policies of various countries
America
The United States is a pioneer in the field of gene cell therapy, with a complete and more conservative and prudent regulatory system. In 2017, FDA approved the first gene therapy product, five years later than the EU. As early as 1984, the United States proposed to regulate the CGT industry, with NIH and FDA conducting double supervision. Since Jesse's death accident occurred in 1999, the United States has strengthened industry supervision, and FDA and NIH have successively issued a number of CGT industry supervision decrees. In 2018, in order to promote the development of the industry, FDA restarted the release of three new guidelines for the development of specific diseases (hemophilia, retinopathy, rare diseases), and updated three existing guidelines to solve production problems related to gene cell therapy.
European union
The European Medicines Agency (EMA) manages CGT according to human drugs in the EU. The gene therapy, cell therapy products and tissue engineering products are defined as Advanced Therapy Medicinal Products (ATMP). The gene cell Therapy is applied for according to the drug application, reviewed by the ATMP committee under EMA, and the review comments are submitted to the CHMP (Committee for Medicinal Produces for Human Use) for final decision, which is finally approved by EMA. So far, the EU has issued a number of guiding principles related to safety supervision.
China
China has carried out basic research and clinical trials in the field of CGT relatively early, regulatory policies and regulations are relatively backward, and the content is relatively simple. The specific issues involved in multiple links of related research and development were not explained and stipulated in detail, the laws and regulations were not strong, and the approval was relatively loose. In 2018, under the influence of "the world's first gene editing baby event", China began to strengthen legislation in biosafety, gene technology, biomedicine and other fields. In 2019, the Biosafety Law and the Regulations of the People's Republic of China on the Administration of Human Resources Genetics were promulgated. In 2020, gene editing and cloning of human embryos into human or animal bodies were included in the criminal law, "If the circumstances are serious, he shall be sentenced to fixed-term imprisonment of not more than three years or criminal detention and shall also be fined; if the circumstances are especially serious, he shall be sentenced to fixed-term imprisonment of not less than three years but not more than seven years and shall also be fined." The Civil Code, which came into force in 2021, also clearly states that "Those engaged in medical and scientific research activities related to human genes, human embryos, etc. shall abide by laws, administrative regulations and the relevant provisions of the State, and shall not endanger human health, violate ethics, or harm public interests." From the regulatory level, it has become stricter and more standardized.











