According to the United Nations Convention on the Rights of the Child, children refer to anyone under the age of 18. The concept of children in China usually refers to people aged 0-14. In 2021, there will be 1.985 billion people aged 0-14 in the world, accounting for 25.33% of the total global population. In 2021, the number of people aged 0-14 in China will be 247 million, accounting for 17.50% of China's total population.
Market scale of children's drugs in China
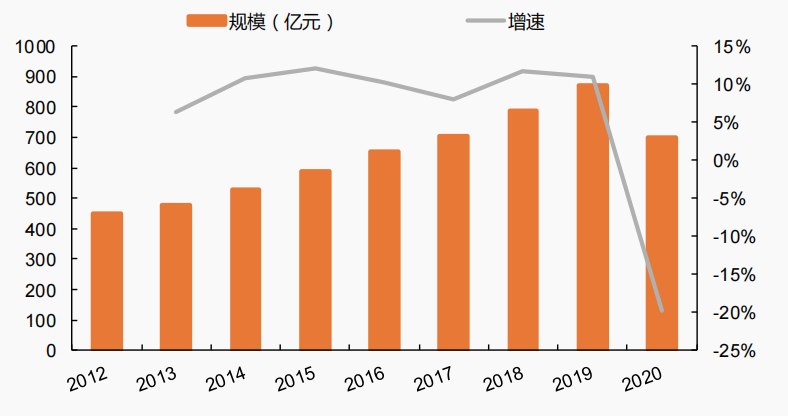
According to the data from Mi Nei Wang, the market size of children's drugs in China reached 87.2 billion yuan in 2019, and the size affected by the COVID-19 dropped to 70 billion yuan in 2020. Over the same period, the total size of drugs in China was about 1.64 trillion yuan, and children's drugs accounted for only about 4% of the total population, which was significantly lower than the proportion of children in the total population. As the impact of the COVID-19 weakens, China's children's drug market will return to the growth trend, and the market size will be about 100 billion in the future.
Figure 1 Market size and growth rate of children's drugs in China from 2012 to 2020
Prevalence rate and visit rate trend of children in China
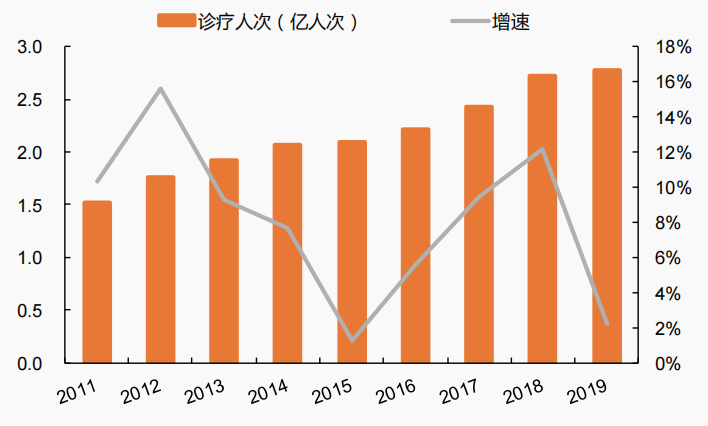
Although the number of newborn babies has declined in recent years, the number of pediatric patients in general hospitals in China has maintained a continuous growth from 2011 to 2019. With the increase of national attention to children's health, and the enhancement of social and family awareness of standardized drug use, the demand for children's drugs in China will continue to release. According to the data of the Health Commission, the two-week prevalence rate and the visit rate of children aged 0-14 have increased, which has driven the continuous increase of children's drug demand. The two-week prevalence rate of children aged 0-4 years increased from 17.4 ‰ in 2008 to 22 ‰ in 2018; The two-week prevalence rate of children aged 5-14 years increased from 7.7 ‰ in 2008 to 13.1 ‰ in 2018. The two-week visit rate of children aged 0-4 years increased from 24.8 ‰ in 2008 to 24.9 ‰ in 2018; The two-week visit rate of children aged 5-14 increased from 9.1 ‰ in 2008 to 11.9 ‰ in 2018.
图 2 2011-2019年我国综合医院儿科就诊人次与增速
Children's medicine ushers in five development opportunities
Policy support continued to strengthen
Since 2011, China has clearly encouraged the R&D and production of children specific drugs, and improved the drug catalog for children. In recent years, children's drugs have received policy support in terms of review and approval, basic drug catalogue, medical insurance catalogue, etc.
Give priority to review and approval to speed up the pace of listing
In 2019, the National People's Congress passed the new version of the Drug Administration Law, clearly encouraging the research and development and innovation of children's drug use, and giving priority to the review and approval of children's drug use. The review time limit of drug marketing license application is generally 200 working days. Compared with the complete application path, the review time limit of priority review and approval procedure is shortened to 130 working days.
Figure 3 Children's drug varieties and approved varieties included in priority review and approval in 2016-2021

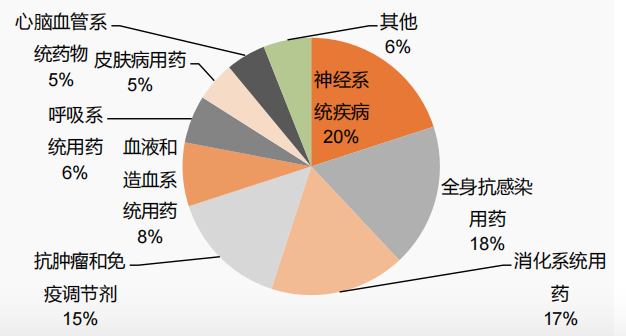
Figure 4 Composition of therapeutic fields of children's drugs with priority for review and approval
Market monopoly helps speed up research and development of children's drugs
In 2022, the State Food and Drug Administration issued the Regulations for the Implementation of the Drug Administration Law of the People's Republic of China (Draft for Comments on the Revised Draft), proposing that the State encourages the research, development, production and innovation of children's drugs. For the first new varieties, dosage forms and specifications for children to be approved for marketing, as well as the increase of children's indications or usage and dosage, a market exclusive period of no more than 12 months will be given, during which the same varieties will not be approved for marketing.
The import of medical insurance catalogue is inclined
Children's medicine is the priority area for the adjustment of the medical insurance catalogue over the years. In 2022, the National Medical Insurance Bureau officially released the Work Plan for the Adjustment of the Drug Catalogue of the National Basic Medical Insurance, Work Injury Insurance and Maternity Insurance in 2022, which appropriately favored rare patients, children and other special groups, and cancelled the restrictions approved after January 1, 2017.
New version of the basic drug catalogue New children's drug catalogue
"Children's medicine" is added to the new edition of the basic medicine catalogue. In November 2021, the Measures for the Administration of the National Essential Drug Catalog (Revised Draft) was publicly solicited for comments, and the "Children's Drugs" catalog was added for the first time within the scope of basic drugs. In 2021, WHO released the eighth edition of the Standard List of Essential Medicines for Children, which collected 29 classifications and nearly 350 varieties. In China's 2018 edition of the List of Essential Medicines for Children, there are only 22 kinds of drugs for children, with huge space potential.
Lindmik Pharmaceutical(Suzhou)Co.,Ltd is a high-tech pharmaceutical enterprise focusing on the research and development, production and sales of innovative pharmaceutical preparations.Equipped with a number of its own innovative R&D platform of dosage forms, including the transdermal drug delivery system, and at the same time, actively introducing the world’s leading nano-based drug delivery, microspheres drug delivery and other cutting-edge pharmaceutical technologies by means of “license in”, the company is a new rapidly developing company pharmaceutical companies that catches people’s eyes.

12th Floor, Building 5, Tianyun Plaza, 111 Wusongjiang Avenue, Guoxiang Street, Wuzhong District, Suzhou City

0512-66020899

0512-66022699

215124