New drugs are usually not only used for the treatment of one disease (first approved indication), but may also be gradually approved for use in other diseases (supplementary indications). A study published in BMJ on July 5, 2023 found that between 2011 and 2020, less than half of the first indications of new drugs approved in the United States and Europe increased the value of substantive Sex therapy compared with the existing treatment, while only about one-third of the supplementary indications increased the value of substantive clinical treatment.
Research Summary
Researchers believe that when primary or supplementary indications cannot provide more benefits than existing treatments, this information should be clearly conveyed to patients and reflected in drug prices. Previously, research on the added value of new drugs was not clear. Therefore, researchers set out to examine all new drugs approved for multiple indications in the United States and Europe between 2011 and 2020, and evaluate the therapeutic value of supplementary indications compared to the first indication.
They used public data to determine 124 primary indications and 335 supplementary indications approved by the U.S. Food and Drug Administration (FDA), and 88 primary indications and 215 supplementary indications approved by the European Medicines Agency (EMA) from January 2011 to December 2020 as analysis samples.
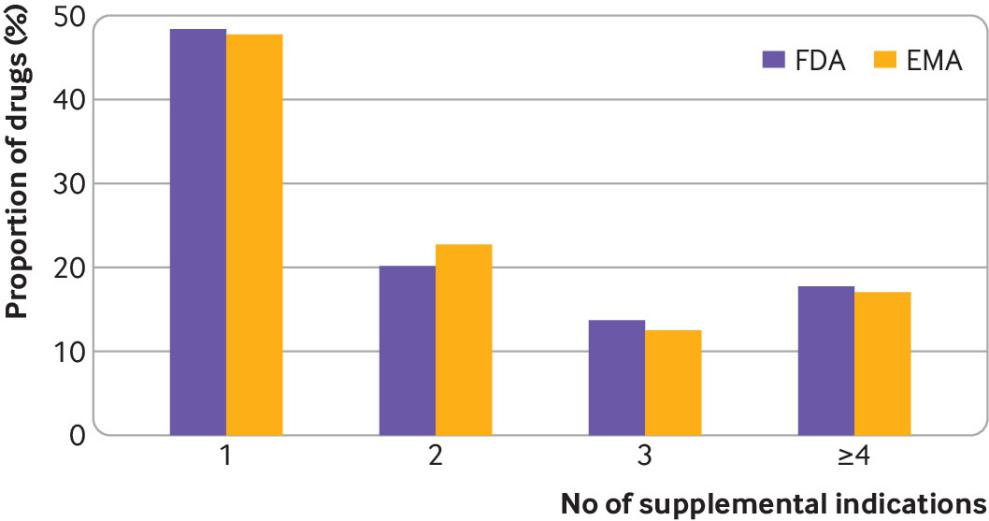
Figure 1: Proportion of drugs approved by the United States and the European Union from 2010 to 21 with one or more supplementary indications.
EMA=European Medicines Agency; FDA=US Food and Drug Administration
In the United States, 48% of drugs have one supplementary indication, 20% have two, 14% have three, and 18% have four or more. In Europe, 48% of drugs have one supplementary indication, 23% have two, 13% have three, and 17% have four or more. The majority (58%) of indications approved by FDA and EMA are for the treatment of various cancers.

Figure 2 The unadjusted percentage of high additional treatment value ratings in the primary and supplementary indications of drugs approved by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA). The reciprocal weighting represents the unadjusted proportion of indications with high therapeutic value.
Among the indications approved by the FDA with available ratings, 41% (44 out of 107) first had a high therapeutic value rating, while the proportion of supplementary indications was 34% (61 out of 179). In Europe, 47% (41 out of 87) of primary indications and 36% (67 out of 184) of supplementary indications have high therapeutic value ratings. In FDA approval, when the sample is limited to the first three approved indications, the likelihood of obtaining a high value rating for the second indication approval is reduced by 36% compared to the first indication approval, and the likelihood of obtaining a high value rating for the third indication approval is reduced by 45%. Similar results have been observed in Europe.
These observations do not cover all drugs, and researchers acknowledge that therapeutic value assessments are not applicable to all indications, especially those approved in the United States but not in Europe. In addition, methods and value assessment systems may be influenced by country-specific factors and assumptions. However, they also pointed out that they focused on the highest rating provided by one of the two health technology assessment institutions, and conducted sensitivity analysis on the value scores of each institution, which confirms the preliminary results. Therefore, their conclusion is:
In the United States and Europe, less than half of approved first-time indications are rated as having high therapeutic value, while the proportion of approved supplementary indications rated as having high therapeutic value is significantly lower than that of approved first-time indications
When indications cannot provide more therapeutic benefits than other available treatment methods, this information should be clearly conveyed to patients and reflected in drug prices
Beate Wieseler of the German Institute for Healthcare Quality and Efficiency stated in a related editorial that new does not necessarily mean better, and needs to be clearly communicated to patients and clinical doctors. The current performance of the system has not met the expectations of patients, the public, clinical doctors, or policy makers, "she wrote. After experiencing the potential of collaborative drug development work during the COVID-19 pandemic, we should seek to align current drug development legislation more closely with established public health goals
To ensure fair pricing and reasonable incentives for innovation investment, a more systematic evaluation of the therapeutic value and cost-effectiveness of supplementary indications is needed. Some Western countries have begun to attempt cross indication weighted pricing or indication based drug pricing. Germany and the UK have implemented weighted pricing, while Switzerland and France have introduced indicator based pricing. Weighted pricing refers to the formulation of a mixed price for a drug based on the value of different indications for each drug. Indication-based pricing is a differential pricing method that prices based on the treatment value provided by the drug for each indication. One rule of Switzerland is that the first price of drugs is the highest possible price, so as the Price ceiling of supplementary indications, the price must be the same or lower. A study suggests that for other countries such as Canada and Australia, as the number of supplementary indications for drugs entering the market increases, the use of management access agreements may also increase, which may help ensure that the value of treatment is consistent with the price paid for further clarification.
Lindmik point of view
The workload of new drug research and development is huge, and the pace of progress is slow, and the period of intellectual property protection is also being consumed. Therefore, many new drugs have chosen the most mature and valuable indications for industrialization as the breakthrough points in the early stage of research and development application; After the product is approved for launch and has accumulated extensive clinical experience, it will be developed and applied for supplementary indications through clinical III trials.
The research results published in the English Journal of Medicine this time are still surprising. The rule of "the waves behind the Yangtze River drive the waves ahead, and a new generation of people trades for the old" does not seem to be applicable to describing the current development status of the pharmaceutical industry. Less than half of new drugs have higher treatment value than old drugs that have been listed in the past, indicating the complexity of human disease evolution and the progress of medical development in postmodern society, To some extent, it also reflects the arduous nature of drug innovation iteration and upgrading, as well as the helpless reality of "pseudo new drugs" and "pseudo innovation" filling in and consuming social medical resources and innovation investment.
Lindmik Pharmaceutical(Suzhou)Co.,Ltd is a high-tech pharmaceutical enterprise focusing on the research and development, production and sales of innovative pharmaceutical preparations.Equipped with a number of its own innovative R&D platform of dosage forms, including the transdermal drug delivery system, and at the same time, actively introducing the world’s leading nano-based drug delivery, microspheres drug delivery and other cutting-edge pharmaceutical technologies by means of “license in”, the company is a new rapidly developing company pharmaceutical companies that catches people’s eyes.

12th Floor, Building 5, Tianyun Plaza, 111 Wusongjiang Avenue, Guoxiang Street, Wuzhong District, Suzhou City

0512-66020899

0512-66022699

215124