
On April 12, 2023, the United States Court of Appeals for the Federal Circuit (CAFC) ruled that the UCB appeal patent was invalid on the grounds of overlapping with the range of existing technology values. This decision indicates that it is difficult to avoid being deemed invalid when there is a partial overlap between the numerical range of the claims and the scope disclosed in the prior art. The following is a report on the content of this judgment.
Background
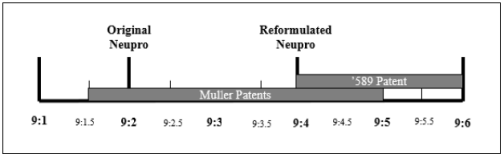
UCB, Inc. (hereinafter referred to as UCB) has manufactured a transdermal patch that can deliver rotigotine to patients' circulatory system. Rotigotine is a drug used to treat Parkinson's disease. This patch contains an amorphous (non crystalline) form of rotigotine, which cannot effectively pass through the skin stratum corneum if the API is in a crystalline state. In 2007, UCB released a new transdermal patch under the trade name Neupro. The initially launched Neupro contains polyvinylpyrrolidone (PVP) to prevent the crystallization of Rotigotine, with a formula ratio of 9:2 for Rotigotine to PVP. Three months after the launch of the first generation Neupro, a new crystal form (Type II) was discovered when Rotigotine was stored at room temperature. This led to the product being recalled in the United States, after which UCB re studied the Neupro process formula and adjusted the ratio of Rotigotine to PVP to 9:4.
Patent
UCB is the owner of US patent number 10130589 (hereinafter referred to as patent 589). Patent 589 protects the weight ratio of rotigotine to PVP from 9:4 to 9:6. The specific claim is as follows: a method for stabilizing rotigotine, which includes an amorphous Solid dispersion containing polyvinylpyrrolidone and rotigotine free base. For example, the weight ratio of rotigotine free base to polyvinylpyrrolidone is in the range of about 9:4 to about 9:6.
In 2013, Actavis Laboratories UT, Inc. (Actavis) submitted a new drug application for Rotigotine transdermal patches to the US Food and Drug Administration (FDA). Since then, UCB has sued Actavis for infringement of its patent 589. In 2021, the United States District Court declared the claims of Patent No. 589 invalid based on foresight. UCB also holds two separate divisional patents [2], patent 589, the comparison of the original Neupro and reconstructed Neupro patents is shown in the following figure:
(1) Novelty
UCB subsequently appealed the judgment of the district court to CAFC. Firstly, CAFC overturned the district court's determination of novelty. The reason is that the local court applied the wrong legal concept when judging the novelty. When judging novelty, if the single point value disclosed in the prior art falls within the range of the claimed values, it does not possess novelty. On the other hand, if existing technology discloses overlapping numerical ranges, it should be "sufficiently clear to enable reasonable factual investigators to conclude that there is no reasonable difference in the operation of the invention within the two numerical ranges." If it only represents the numerical range of the main complaint, it does not possess novelty.
According to CAFC, UCB patents disclose overlapping numerical ranges. However, the local court did not adopt the traditional approach of analyzing overlapping numerical ranges, but instead treated each point falling within that range as a point within the claimed numerical range for analysis. The court held that the broad range of 9:1.5 to 9:5 in the patent resulted in data discreteness within the publicly claimed numerical range (such as 9:4 and 9:5), and was deemed unfair in the patent.
(2) Obvious
CAFC upheld the district court's obvious ruling regarding the claims of UCB patent 589 and stated the following reasons.
(2-1) Overlapping numerical range
CAFC points out that when the numerical range of the claims overlaps with the disclosed range in the prior art, it is presumed that they are obvious. CAFC also believes that existing technology poses obstacles to the required numerical range, resulting in new and unexpected results or other evidence related to the required numerical range. If it exists, it is said that the presumption can be overturned. Firstly, it argues that the overlap between the numerical range of claims in patent 589 and the numerical range of publicly available patents is undisputed, and Actavis presented an obvious evidence.
(2-2) Obstructive factors
Even if there is no conflict between the overlapping numerical ranges mentioned above, UCB's more publicly available patents were written before the discovery of Form II of Rotigotine, and cannot reflect the technical level at the time of the invention. I attempt to refute it by arguing that it is not obvious. Especially when examining UCB patent 589, the examiner found that it was closest to the Tang [3] reference patent and believed that the reference patent was not conducive to the numerical range of the claims.
However, CAFC did not accept this objection. CAFC pointed out that the crystallization of rotigotine occurs through hydrogen bonding between two rotigotine molecules. Given the similarity between crystal form I and crystal form II, the ruling of the local court was not incorrect, that is, there was no necessary prerequisite that made the existing technical documents of crystal form II unusable.
The UCB further assumes that Tang has hindrance factors in response to this accusation. The Federal Circuit Court argues that the citation only states that other inventions are more desirable and does not refute, challenge or prevent research on the claimed invention, and cannot be said to have hindrance factors. Although Tang stated that 9:18 is the preferred ratio for long-term stability, he did not say anything to prevent technicians in this field from using the range of 9:4 to 9:6. In particular, the Federal Circuit cited previous judicial precedents stating that one viewpoint is preferred and does not refute, doubt, or hinder the study of other viewpoints.
(2-3) Unexpected results
Whether UCB can prove unexpected effects has also been refuted by CAFC. The evidence of unexpected effects must indicate that there is a difference between the invention and the closest existing technology, and that the difference is unexpected for those skilled in the art. CAFC also pointed out that degree differences are not as important as category differences, and good therapeutic effects may bring unexpected benefits.
UCB emphasizes that patches with a weight ratio of 9:4 to 9:6 between Rotigotine and PVP have unexpected effects that do not cause crystallization. This unexpected effect originated from the only patch within the patent disclosure scope of UCB's first generation Neupro (with a weight ratio of 9:2 rotiguptin to PVP), and crystallization occurred in the first generation Neupro. If so, they believe that people would expect crystallization to occur throughout the entire patent range (9:1.5 to 9:5). CAFC believes that such arguments are not convincing, and the fact that crystallization occurred in the first generation Neupro (9:2) provides technical personnel in this field with the full range of publicly available patents (9:1.5 to 9:5). Its conclusion is that it will not lead to recognition of crystallization.
Here, CAFC referred to expert testimony and existing technology, and (i) as of 2009, the chemical background of the Rotigotine/PVP reaction has been fully understood, and PVP has been validated as the most effective crystallization inhibitor. (ii) There is evidence to suggest that increasing the amount of PVP can make the formulation more stable.
(2-4) Commercial Success
Finally, CAFC refuted UCB's claim that Neuropo had achieved commercial success through redevelopment, optimization, and re launch. The court argued that there were many reasons for the product's commercial success, and commercial success alone did not prove that the claimed invention was not obvious. In this case, it is believed that the litigation patent is an obstacle to preventing competitors from developing a treatment system for Toupilotidine. From previous judicial precedents, the possibility of commercial success as evidence to infer non obviousness is very low in cases where there are barriers to the entry of patents that hinder other enterprises.
(2-5) CAFC Summary
In summary, CAFC refutes UCB patents one by one without obvious grounds for protest, and maintains the district court's judgment on patent invalidity.
The ruling provided several responses to the patentee's apparent determination in cases where the numerical range of the claims partially overlaps with the scope disclosed in the prior art. UCB refuted the disputed points, but was not adopted. It indicates that if the specific numerical range of the inventor's patent request overlaps with the existing technology, there is a high risk of being challenged and invalidated by the patent.
The judgment text described in this case (CAFC judgment) can be obtained from the following website.
https://cafc.uscourts.gov/opinions-orders/21-1924.OPINION.4-12-2023_2109643.pdf
Lindmik Pharmaceutical(Suzhou)Co.,Ltd is a high-tech pharmaceutical enterprise focusing on the research and development, production and sales of innovative pharmaceutical preparations.Equipped with a number of its own innovative R&D platform of dosage forms, including the transdermal drug delivery system, and at the same time, actively introducing the world’s leading nano-based drug delivery, microspheres drug delivery and other cutting-edge pharmaceutical technologies by means of “license in”, the company is a new rapidly developing company pharmaceutical companies that catches people’s eyes.

12th Floor, Building 5, Tianyun Plaza, 111 Wusongjiang Avenue, Guoxiang Street, Wuzhong District, Suzhou City

0512-66020899

0512-66022699

215124